Quality Management
Data accuracy is essential.
See how the QRS system can provide it.
The key to any successful clinical trial is having reliable and accurate data entered in a timely fashion for sponsors and CROs. We strive to support study data collection with particular care for the volunteers while leveraging standard practices and guidelines tailored to your business needs.
We understand your business. We’re experienced with standard operating procedures and quality management plans. Our extensive industry knowledge goes into everything we do.
Our goal is simple:
Meet sponsor demands while
improving workflow efficiency and quality
The growing trend to risk-based monitoring means less interaction with monitors. And that leads to greater focus on maintaining a quality system at your site.
Good quality systems do a few key things:
- Involve the right staff at the right points
- Help improve current practices without being prompted by corrective and preventive actions (CAPAs)
- Reduce queries to create an effective solution against errata
We’ll start with a diagnostic of your current QA methodology and create a customized system that’s right for your site.

“What gets measured gets managed.”
– Peter Drucker, The father of modern management
How Your Business Benefits
Reliable & accurate data, entered in a timely fashion
SOP’s, templates and forms ready to enhance the quality of your data collection
The ability to perform perform study audits on a regular basis
Improved staff collaboration with a simultaneous use
Key Features
Quality Planning Capabilities
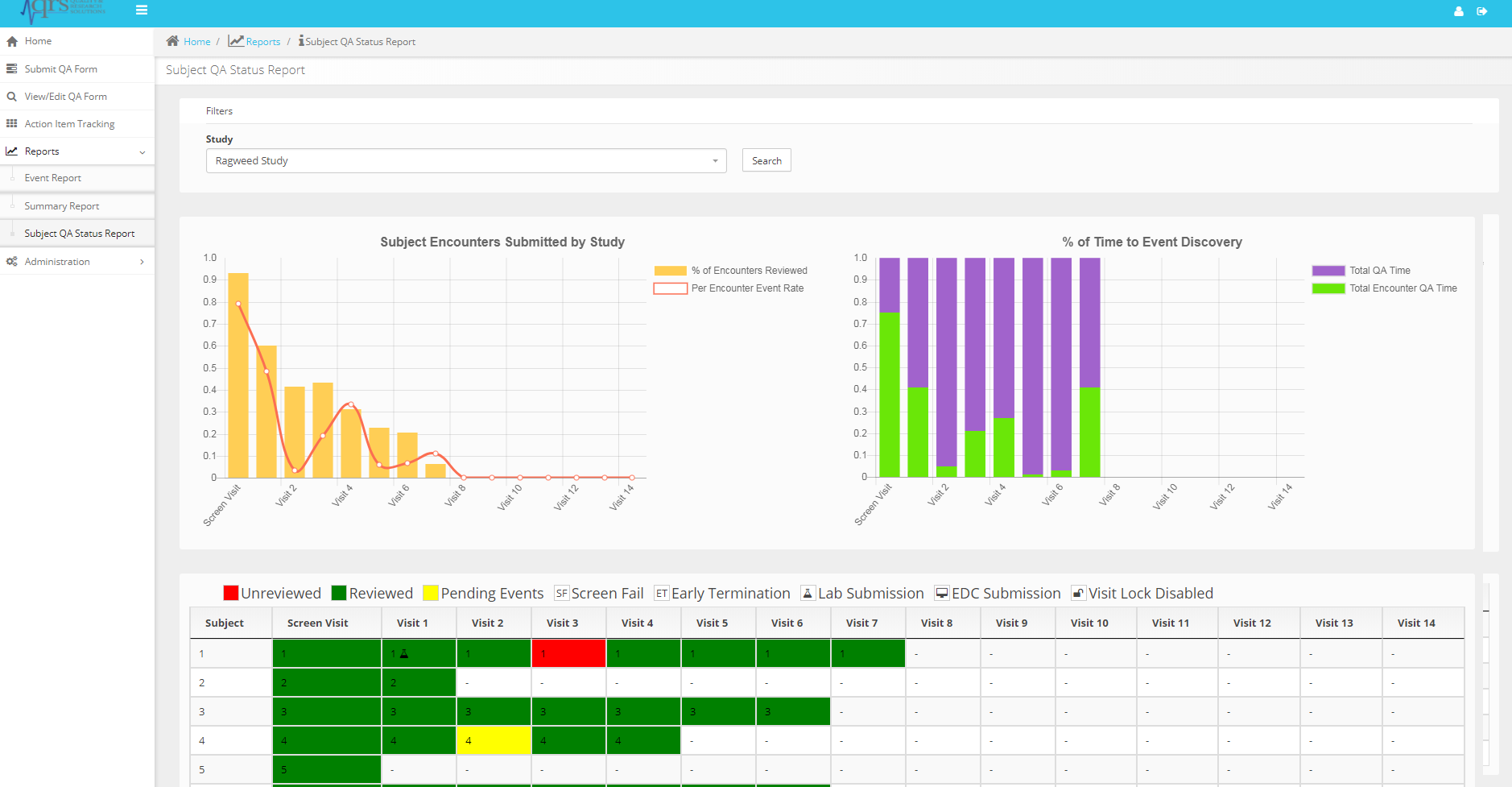
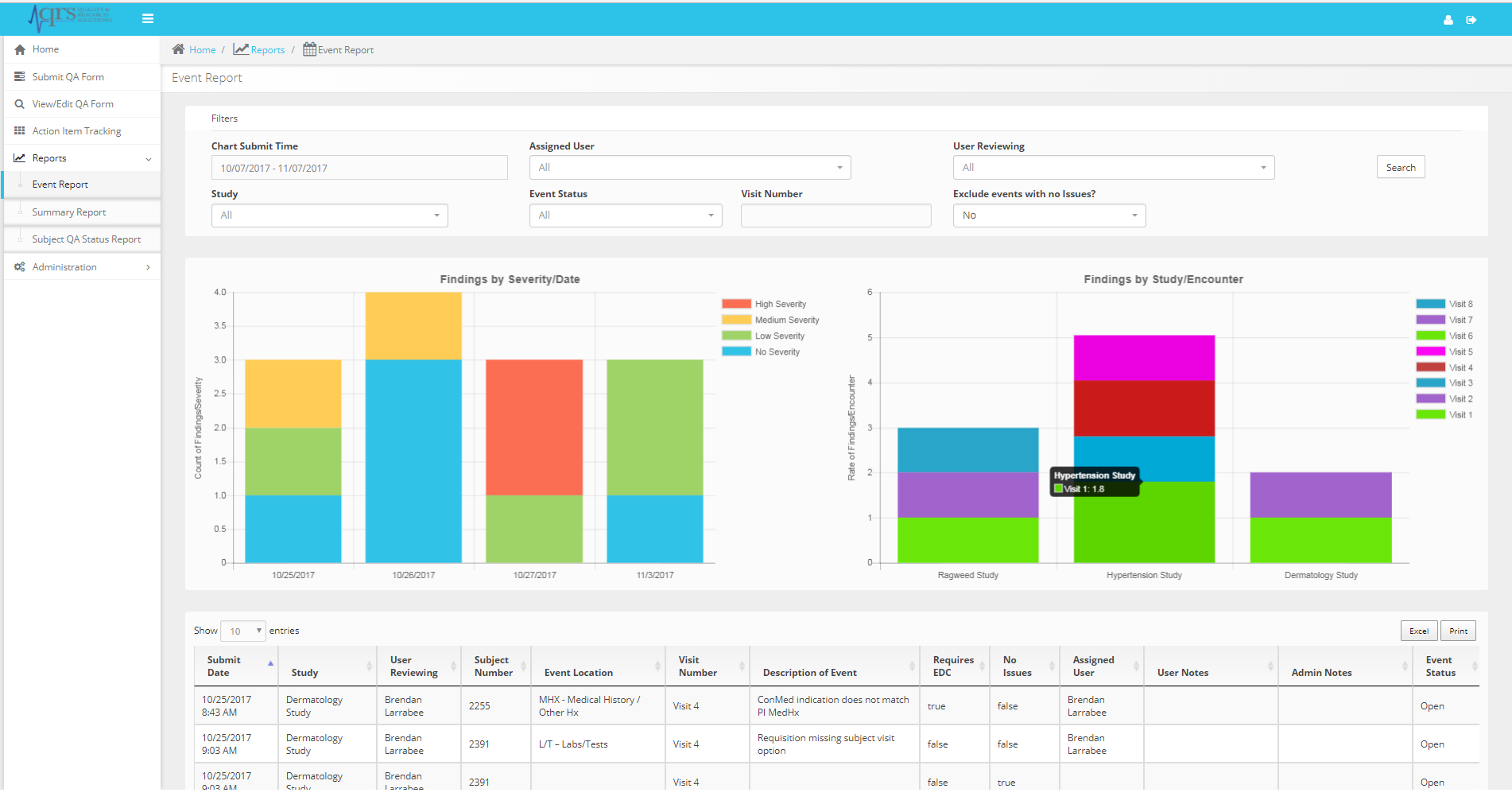
Studies generate a large amount of data and many opportunities to improve quality. This application provides standard reports and graphs to help you make the right decisions when it comes to ensuring study quality. Plan the workload appropriately based on scope and risk to data integrity.
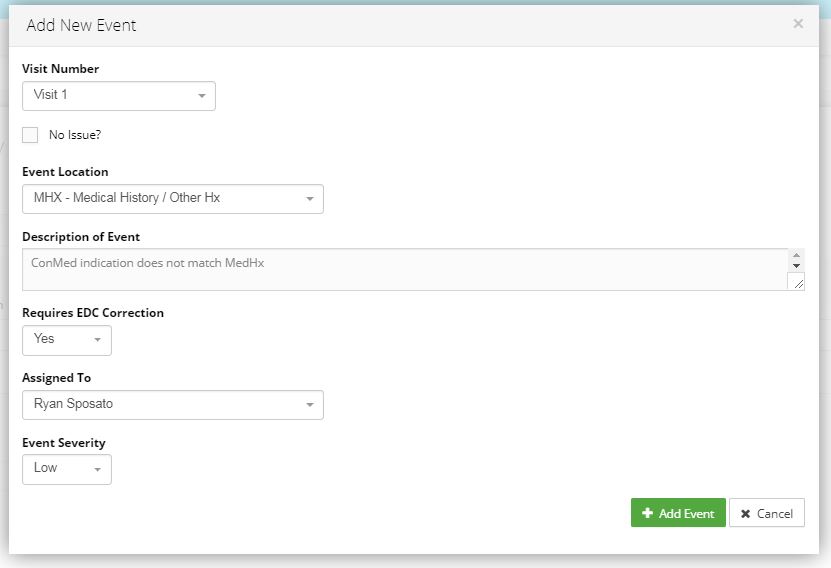
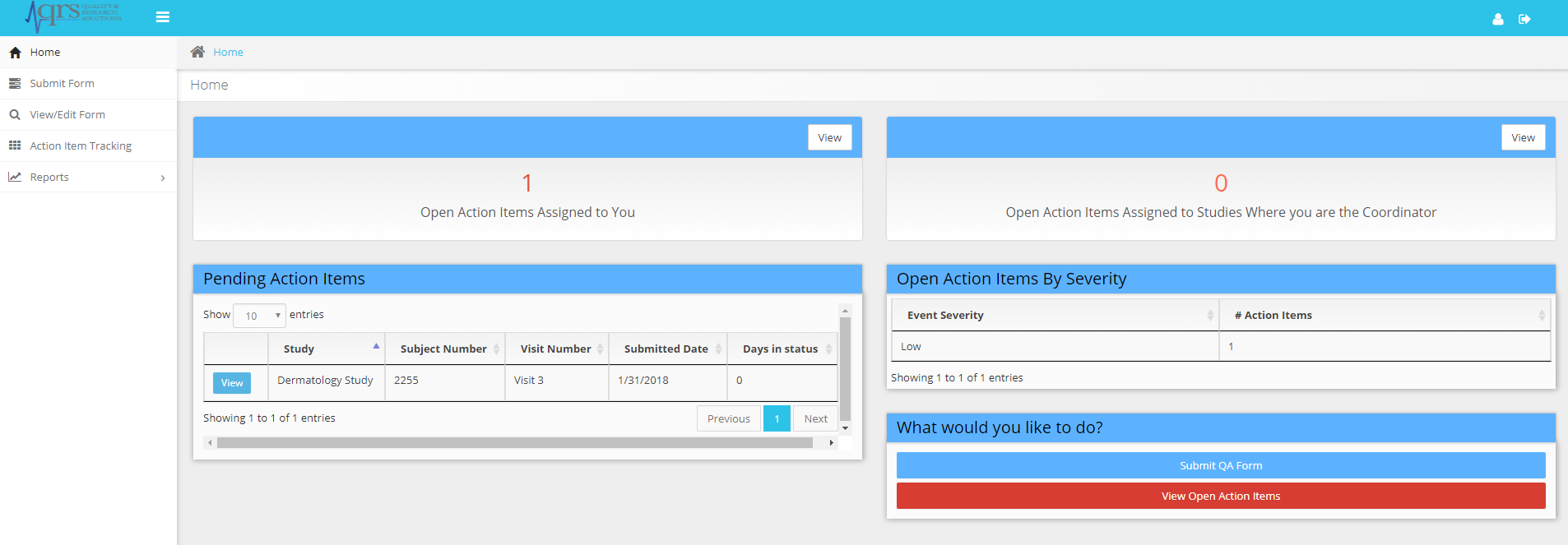
Action Item Tracking System
Manage submissions and get real time notification of corrective action needed based on user roles. Enable your staff to address findings and inform management of progress leads to a quick turnaround for data corrections.
The Action Item Tracking system and email notifications (click to zoom)
Frequently Asked Questions
About the Software
How will this give me an advantage over my competitors?
- Quality data leads to more business – metrics for error rates are tracked by sponsors and affect selection for future work.
- Better data for sponsors and identifying gaps in training, processes or documentation.
How does this help me?
- Automates quality processes that were manual.
- Auto emails, don’t need to track down with chart, faster reaction to issues and know the trends and fixes are being addressed.
- Supplements sponsor movement to risk based monitoring while increasing data sharing.
How does it help me improve my site?
You need data to shed light on where the errors are hiding. Finding the root cause will allow you to find the solutions to removing them.
- Increase staff accountability and transparency, without the stigma.
- Follow the effectiveness of your site’s quality program with increased study coverage and better audit planning.
What does it do?
- Automates the process and sends email to the person who needs to fix the error.
- Creates reports identifying trends, allows the severity ranking of the errors.
- Works with existing processes without too much set up.
How does this system compare to e-source solutions?
E-Source is intended to reduce the most common mistakes made by clinical staff such as transcription errors, invalid or inconsistent data. While they may capture a vast majority of common errors, we are all human and more complex issues may not be found as easily.
Who on my staff would use it?
Everyone – primarily quality, the SC, Research Assistants but, all can have access. PI and Admin particularly like to see the trends and reports.
Does this save me time? If so how?
Yes – old process was find an error take the chart to the person who made the error, wait around so chart is not misplaced, fix the error manually enter into tracking log that error was fixed. Now the person is notified by email and change can happen live in the tracking tool.
Is this more work for me and my staff?
By eliminating rework identified in an active quality assurance program you are saving future time and effort. Especially if you don’t have QA or are doing it manually!
About QRS
How has this tool changed how we do things?
We have instituted new processes for review of source – eliminated some of the poor design features that led to error, increased awareness of the importance of doing it right the first time.
What have other sites learned since using it?
There were higher error rates at critical visits – v1 and randomization visits. Process and documentation changes were needed to bring that rate down!
Why did we create this software? Why do we need this?
- To create and foster a culture of quality and teamwork.
- We needed this to automate the collection of data and analyze trends leading to solutions.